Why Does Potassium Have a Low Ionization Energy
Why does potassium have lower ionization enthalpy than AR. The alkaline earth metals form oxide in the earth and they are soluble in water.

Alkaline Ionized Water Explained Alkaline Water Kangen Water Ionised Water
And thats what happens say dont do this if you put a chunk of potassium metal into water.

. Simply put aluminum has 3 valence electrons while sodium only has 1. Does calcium have a high or low electronegativity. Potassium has a low ionization energy.
This means that less energy is needed to remove the outermost electron and therefore the ionisation energy is lower. Answered by Abida R. Why is the first ionization energy of potassium less than sodium.
This allows Chlorine to have a greater pull or electronegativity that Sodium It also has a smaller atomic radii due to the greater pull Sodium has a higher electronegativity than Potassium. Electron affinities are more difficult to measure than ionization energies. On this page we have gathered for you the most accurate and comprehensive information that will fully answer the question.
Ionization energy also called ionization potential is the energy necessary to remove an electron from the neutral atom. So atomic number of potassium is 19 and it has configuration of Ar 4s1. The greater distance means that the attraction between the electron is weaker.
K e K H Affinity 484 kJmol To use electron affinities properly it is essential to keep track of sign. As a result more energy will be required to pull the electron away from the. Because the outer electron in lithium is at a greater distance from the nucleus and experiences a smaller attraction for the nucleus than the electrons in an He atom it takes less.
It takes more energy for aluminum to lose 3 electrons to complete its octet shell than it. In chemistry it often refers to one mole of a substance molar ionization energy or enthalpy and is reported in kJmol. Why does gallium have a low ionization energy.
Why would something have a low ionization energy. Which is greater the second ionization energy of potassium or that of calcium. Bones are mostly calcium phosphate.
And if its easy to lose an electron you would expect it to be really reactive - say to lose that electron really quickly. The electron in Potassium is also more affected by shielding due to more shells further weakening this attraction. There is more shielding between the nucleus and the outer electrons and the distance between the nucleus and the outer electron increases and therefore the force of attraction between the nucleus and outer most electrons is.
From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. Is potassium alkaline earth metal. Looking for an answer to the question.
Looking for an answer to the question. First Ionization Energy of Potassium is 43407 eV. Ionization enthalpy is defined as amount of energy required to remove the outermost electron from a gaseous isolated atom.
Why is the first ionization energy of potassium less than sodium. The alkali metals are Beryllium Be Magnesium Mg Calcium Ca Strontium Sr Barium Ba and Radium Ra. Calcium chloride reacts with potassium phosphate to form calcium phosphate and potassium chloride.
According to periodic table the elements Zinc Gallium and Potassium belongs to 4th period. Potassium has a low ionization energy telling you that its really easy to lose an electron. Write out the electron configurations.
This is the reason they are named so. Both Sodium and Potassium are in the same family. Calculate the ionization energy for an atom of hydrogen making the assumption that ionization is the transition from n1 to ninfinity.
Electronegativity increases across a period. Why does gallium have a low ionization energy. Which element has the lowest ionization energy and why.
0 Apr 28 2017 4 Ok thanks. Decreases the ionization energy of O atom compared to N atom. In atomic physics the ionization energy is typically measured in the unit electron volt eV.
An atom of Potassium in the gas phase for example gives off energy when it gains an electron to form an ion of Potassium. What element has the. Potassium is greater in the second ionization energy.
Large atoms or molecules have low ionization energy while small molecules tend to have higher ionization energies. Why is the first ionization energy of potassium less than sodium. Calcium is higher in the group than barium so will have a higher electronegativity.
Sodium has only 11 protons pulling on its electron density while Chlorine has 17 protons. Going down the group the first ionisation energy decreases. Jul 24 2017 Because nitrogen does not have any paired valence 2p electrons that can be removed while oxygen does.
Does sodium have a higher ionization energy than aluminum. Why does gallium have a low ionization energy. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group.
But the ionization energy of gallium is lower than zinc because Zinc has a completely filled electronic configuration whereas gallium which starts filling p-orbitals. On this page we have gathered for you the most accurate and comprehensive information that will fully answer the question. Coulombic repulsion energy decreases the energy input required to remove a 2p valence electron from O atom ie.
Click to see full answer. The lowest value will therefore be in the bottom left of these atoms thats caesium. Why does lithium have a low ionization energy.
Electronegativity increases as you go up a group. The ionization energy is different for electrons of different atomic or molecular orbitals. What is the ionization energy of gallium.
Why does gallium have a low ionization energy. Hence they have low ionization energy. Conversely as one progresses down a group on the periodic table the ionization energy will likely decrease since the valence electrons are farther away from the nucleus and experience greater shielding.
Potassium - Ionization Energy.

Why Choose Himalayan Natives Pink Salt Himalayan Pink Salt Healthy Salt Pink Salt
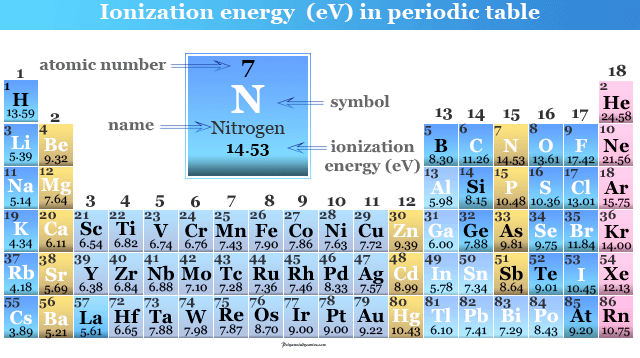
Ionization Energy Definition Equation Periodic Table Trends

Lesson Explainer Ionization Energy Nagwa

Alkamate Portable Alkalizer Water Bottle With Filter Water Ionizer Alkaline Water Water Purification

The Parts Of The Periodic Table

Pin On Small Intestine Problem

What S In Your Cup 2015 Edition Cup Food Chemistry Science Certificates

The Parts Of The Periodic Table
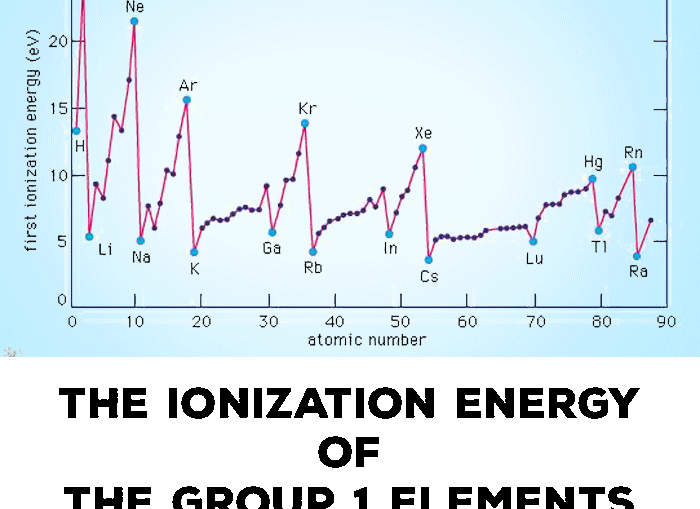
Ionization Energy Or Ionisation Energy Of Group 1 Alkali Metals Elements Tuition Tube
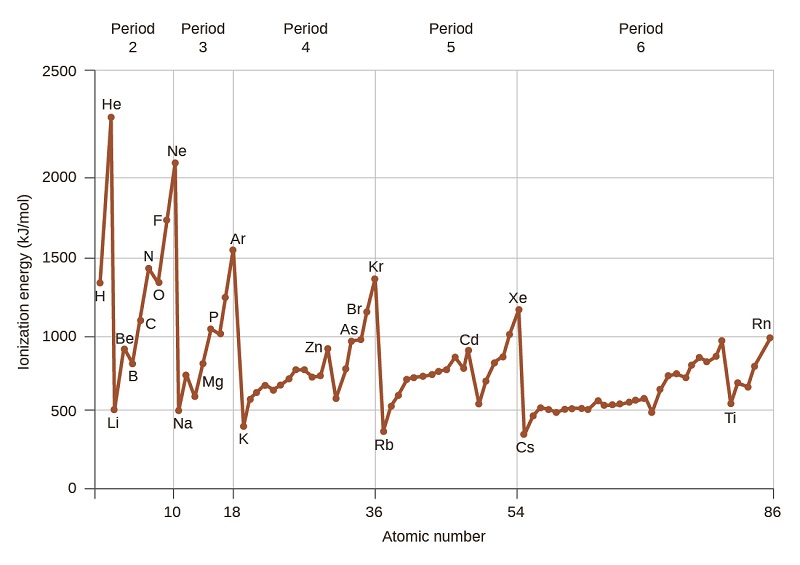
3 3 Trends In Ionization Energy Chemistry Libretexts

This Shows Some Of The Elements Ionic Radius Potassium Is K The Ionic Radius Is 133 Ionic Radius Elements Teaching Science

Why Does Potassium Has Lower Ionization Enthalpy Than Ar Quora

Lesson Explainer Ionization Energy Nagwa

Bromine Facts Atomic Number 35 And Element Symbol Br Element Symbols Ionization Energy Atomic Number

Periodic Trends In Ionization Energy Ck 12 Foundation

Ionization Energies Quizzes Chemistry Quiz 16 Questions And Answers Practice Chemistry Quizzes Based Questio Energy Quiz College Chemistry Ionization Energy

Explained 5 Factors Affecting Ionization Energy And Its Trend

The Importance Of Magnesium On Keto Keto Diet Food List Keto Breath Keto Diet Recipes

Comments
Post a Comment